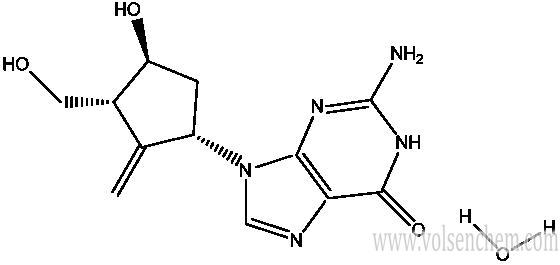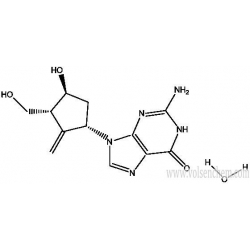


Entecavir monohydrate(209216-23-9) is a nucleoside analog(more specifically, a guanine analogue) that inhibits reverse transcription, DNA replication and transcription in the viral replication process.
Thera. Category: Adult chronic hepatitis B
Cas No.:209216-23-9
Synonym:entecavir hydrate;ENTECAVIR MONOHYDRATE;2-Amino-1,9-dihydro-9-[(1S,3R,4S)-4-hydroxy-3-(hydroxymethyl)-2-methylenecyclopentyl]-6H-purin-6-one monohydrate;Entecavir hydrate / 2-Amino-1,9-dihydro-9-[(1S,3R,4S)-4-hydroxy-3-(hydroxymethyl)-2-methylenecyclopentyl]-6H-purin-6-one monohydrate
Molecular Formula:C12H17N5O4
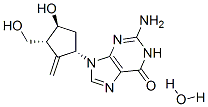
Molecular Weight:295.29448
Pharmacopeia: In house Spec.
Specifications:Available on request
Packing:Export worthy packing
Material Safety Data Sheet:Available on request
Usage:This product is applicable to the treatment of adult chronic hepatitis B.
|
Product Name |
Entecavir Monohydrate |
Cas No. |
209216-23-9 |
|
Producing Date |
2014-10-03 |
Batch No. |
20141003 |
|
Assaying Date |
2014-10-03 |
Reporting Date |
2014-10-03 |
|
Expiry Date |
2016-10 |
Quantity |
50Gram |
|
Items |
Specifications |
Results |
|
|
Appearance |
White or off-white powder |
White powder |
|
|
Identification |
1)The IR absorption specturm of the sample must be same with entecavir 2) RT of the sample should match that of standard assay preparation |
Comform |
|
|
Water |
5.8%~6.5% |
6.1% |
|
|
Optical rotation |
+24°~+28° (DMF:MOH=1:1 C=1%) |
+27.2° |
|
|
Residue on ignition |
≤0.10% |
0.07% |
|
|
Heavy Metals |
≤20ppm |
Conforms |
|
|
Related substances |
Total impurities |
≤0.30% |
0.07% |
|
Single impurity |
≤ 0.1% |
0.03% |
|
|
Residual solvents |
dichloromethane |
≤600ppm |
100PPM |
|
methanol |
≤1000ppm |
300PPM |
|
|
tetrahydrofuran |
≤700ppm |
ND |
|
|
toluene |
≤600ppm |
ND |
|
|
Assay (HPLC) |
99.8% ~102.0%(On anhydrous basis) |
≥99.9% |
|
|
Particle size |
D90≤30um |
Conform |
|
|
Conclusion: The product corresponds to the requirements of in house standard. |
|||